YOUR DAILY ASSEMBLE AND PRODUCTIVITY COMPANION
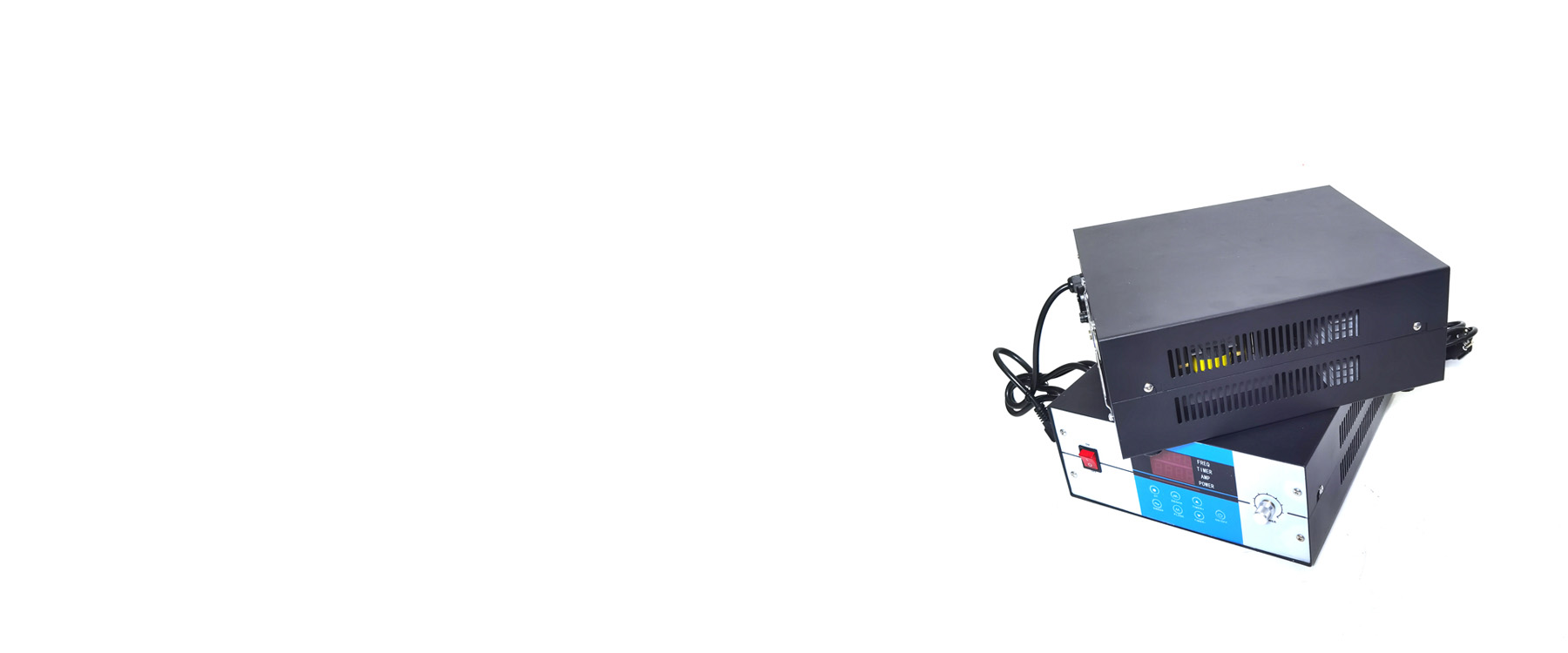
SKSONIC is a leading in the ultrasonic technology research and development. The main products include ultrasonic generator, ultrasonic transducer, ultrasonic cleaner, ultrasonic welder and other ultrasonic core accessories.
Our SKSONIC products mainly applied in ultrasonic cleaning equipment, ultrasonic welding equipment, ultrasonic cutting equipment and other customized ultrasonic application.All of our products are CE/FCC certificated.
ULTRASONIC AND PIEZOELETRIC
SKSONIC Meticulous in aspects from ultrasonic research and development, product strategy to supplier management, SKSONIC team has been pursuing superior quality, concentrating on creating easily-operated, maintanience convenient, price moderate and quality reliable products to deliver you a delightful product experience.
PRODUCT WARRANTY
Ultrasonic and piezo short lead time for both standard products(3-5days) and customized products(10-15days). One year warranty, free technical support all lifetime.
With 100% detection coverage, you can tap anywhere on the large, precision-engineered and be sure of powerfully responsive control.
-
Amazon E-commerce
Amazon.com is a Fortune 500 e-commerce company based in Seattle, Washington, and has the distinction of being one of the ... -
Alibaba Launches A100 Strategic Partnership Program
The A100 initiative was announced at the inaugural “Alibaba ONE Business Conference” in Hangzhou. The name, “A100,” symbolizes Alibaba’s goal ... -
Amazon E-commerce
Amazon.com is a Fortune 500 e-commerce company based in Seattle, Washington, and has the distinction of being one of the ... -
Alibaba Launches A1002 Strategic Partnership Program
The A100 initiative was announced at the inaugural “Alibaba ONE Business Conference” in Hangzhou. The name, “A100,” symbolizes Alibaba’s goal ...
 Ultrasonic Transducer,Ultrasonic Generator,Ultrasonic Cleaner -SKSONIC
Ultrasonic Transducer,Ultrasonic Generator,Ultrasonic Cleaner -SKSONIC